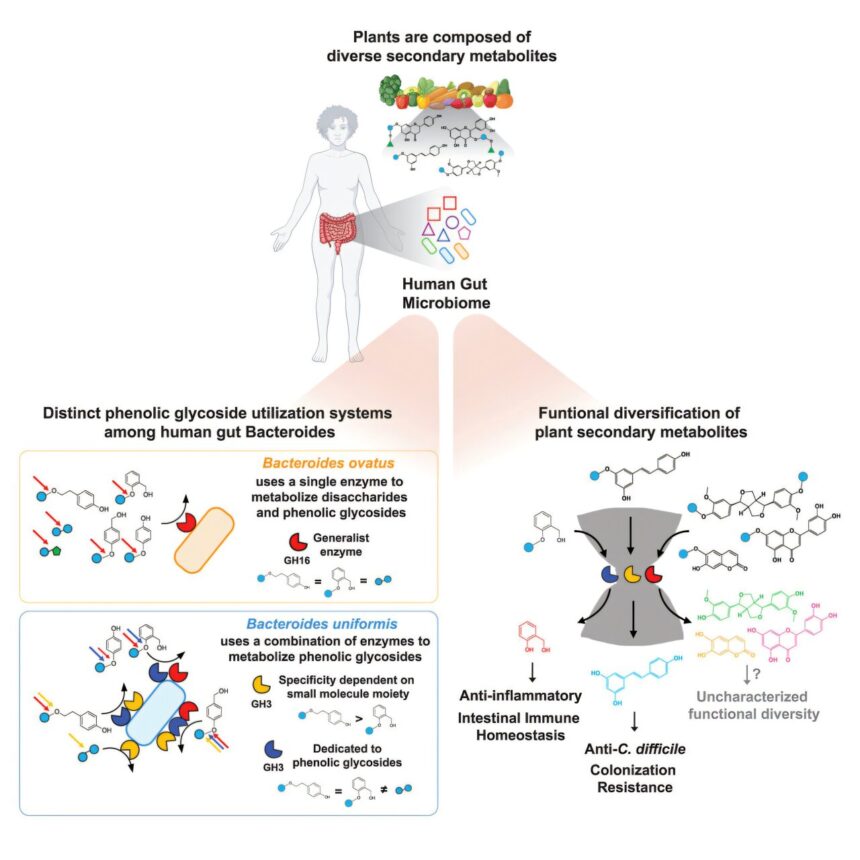
Nutrition is a vital part of our daily lives, yet it remains somewhat of a mystery. While we know that many plant-based foods are beneficial for our health, the role of our intestinal microbiome in breaking down these foods is still not fully understood. A recent study published in the journal Cell sheds light on how we can optimize our diets by leveraging our gut bacteria to metabolize plant compounds known as phenolic glycosides.
Lead researcher Seth Rakoff-Nahoum, MD, Ph.D., from Boston Children’s Hospital, explains that phenolic glycosides are plant compounds that consist of sugar molecules bound to various small molecules with potential health benefits. By studying how different intestinal microbes use specialized enzymes to break down these compounds, the research team identified specific small molecules that can help regulate intestinal inflammation and enhance resistance to intestinal pathogens.
The study focused on Bacteroides, a prominent group of bacteria in the human gut microbiome, and investigated how 52 strains of Bacteroides and Parabacteroides metabolized seven phenolic glycosides. The researchers found that certain small molecules released by these bacteria’s enzymes could inhibit the colonization of intestinal pathogens like Clostridioides difficile. For example, resveratrol, derived from polydatin found in grapes and red wine, exhibited antibiotic properties against C. difficile in mouse models.
Another compound, salicin, extracted from willow bark, was shown to have anti-inflammatory effects when metabolized by Bacteroides in the intestine. This process released saligenin, which helped maintain intestinal balance and regulate the immune response. The researchers believe that these findings could lead to new therapeutic approaches for conditions like inflammatory bowel disease and C. difficile infections.
Moving forward, the research team aims to explore the clinical implications of their discoveries by potentially developing treatments that combine plant phenolic glycosides with bacterial enzymes or bacteria themselves. By harnessing the power of our microbiome to enhance the effects of dietary compounds, there is hope for the development of novel therapies for gastrointestinal diseases.
In conclusion, the study published in Cell opens up a new field of research with significant therapeutic potential. By understanding how our gut bacteria metabolize plant compounds, we can unlock the health benefits of our diets and potentially revolutionize the treatment of gastrointestinal conditions. The collaboration between diet and intestinal microbes could pave the way for a healthier future.